The DIRECT project was designed to explore some of these differences, to work out why people don’t react in the same way. We will be able to see whether people with Type 2 diabetes are similar and if they have the same experience or not. This will help predict how other people might respond.
The studies were designed to identify if there are signals, called ‘biomarkers’, which might flag whether particular patients belong to different sub-groups. These sub-groups are based on each patient’s experience of Type 2 diabetes. These biomarkers can then l be used to help diagnose people with Type 2 diabetes, or to help choose the best treatment pathways for them.
The project was split into two areas:
- Studying the way that Type 2 diabetes progresses in some patients.
- Studying the way that different patients respond to different treatments.
The research was funded by the European Union, with funding from some leading pharmaceutical companies. Together, this group of European researchers and scientists is called the DIRECT Consortium. DIRECT is interested in learning about the risk factors for diabetes and how people with diabetes begin their diabetes journey or respond to treatment.
The DIRECT study was divided up into nine different “Work Packages” – WP1 to WP 9.
The DIRECT Work Packages
The DIRECT consortium was organized in a highly integrative and synergistic framework dividing the project in two consecutive parts with contributions of seven strongly interrelated scientific work packages as shown in the figure below:
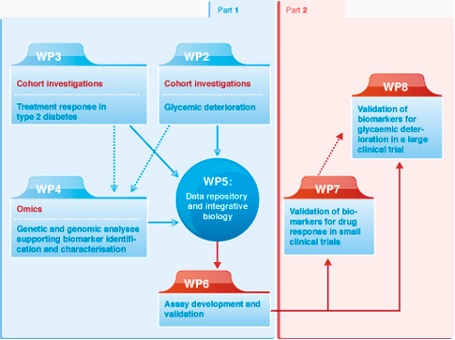
Work packages 1 and 9 were management work packages, with WP9 specifically designed to address the ethical and legal requirements of a large data-repository of pan-European data, and the specific issues related to treatment stratification. WP1 addressed the aspects of project management and administration.
The seven interrelated research work packages can broadly be split into phenotype generation and provision (WP2 and 3), data generation and analysis (WP4 and 5), validation and assay development (WP6). Validation of the findings were planned via through prospective clinical trials (WP7 and 8).



